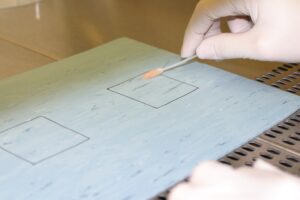
Wir führen seit vielen Jahren Desinfektionsmittelprüfungen nach anerkannten Prüfmethoden durch.
Dazu zählen unter anderem:
- Quantitative Suspensionsteste
- Praxisnahe Prüfmethoden wie z.B.:
- 4 Feldertest zur Prüfung von Flächendesinfektionmitteln mit Mechanik
- Keimträgertest zur Prüfung von Flächendesinfektionmitteln ohne Mechanik
- Praxisnahe Händetests (Händewaschung oder Händedesinfektion)
- Keimträgertest zur Prüfung von Instrumentendesinfektionmitteln
- Prüfungen nach DIN 13063
Gerne entwickeln wir mit unseren Auftraggebern zusammen neue Prüfmethoden oder Problemlösungen im Bereich hygienischer Sicherheit, die an die speziellen Anforderungen vor Ort zugeschnitten sind. Wir beraten Sie gerne.
Wir sind nach DIN EN ISO/IEC 17025 nach DIN EN ISO 15189 akkreditiert.
Zudem sind wir ein anerkanntes Testlabor des VAH.

Ansprechpartner

PD Dr. rer. nat. Maren Eggers
- eggers@labor-enders.de
- +49 (711) 6357-130
- +49 (711) 6357-138
- Virologie, Desinfektionsmitteltestung, Angewandte Hygiene
Auswahl unserer Publikationen/Beiträge:
Kramer, A; Arvand, M; Christiansen, B; Dancer, S; Eggers, M; Exner, M; Müller, D; Mutters, NT; Schwebke, I; Pittet, D
In: Antimicrobial resistance and infection control, Bd. 11, S. 93, 2022.
@article{Kramer.2022,
title = {Ethanol is indispensable for virucidal hand antisepsis: memorandum from the alcohol-based hand rub (ABHR) Task Force, WHO Collaborating Centre on Patient Safety, and the Commission for Hospital Hygiene and Infection Prevention (KRINKO), Robert Koch Institute, Berlin, Germany},
author = {A Kramer and M Arvand and B Christiansen and S Dancer and M Eggers and M Exner and D M\"{u}ller and NT Mutters and I Schwebke and D Pittet},
doi = {10.1186/s13756-022-01134-7},
year = {2022},
date = {2022-07-06},
urldate = {2022-07-06},
journal = {Antimicrobial resistance and infection control},
volume = {11},
pages = {93},
abstract = {BACKGROUND
The approval of ethanol by the Biocidal Products Regulation has been under evaluation since 2007. This follows concern over alcohol uptake from ethanol-based hand rubs (EBHR). If ethanol is classified as carcinogenic, mutagenic, or reprotoxic by the European Chemicals Agency (ECHA), then this would affect infection prevention and control practices.
AIM
A review was performed to prove that ethanol is toxicological uncritical and indispensable for hand antisepsis because of its unique activity against non-enveloped viruses and thus the resulting lack of alternatives. Therefore, the following main points are analyzed: The effectiveness of ethanol in hand hygiene, the evidence of ethanol at blood/tissue levels through hand hygiene in healthcare, and the evidence of toxicity of different blood/tissue ethanol levels and the non-comparability with alcoholic consumption and industrial exposure.
RESULTS
EBHR are essential for preventing infections caused by non-enveloped viruses, especially in healthcare, nursing homes, food industry and other areas. Propanols are effective against enveloped viruses as opposed to non-enveloped viruses but there are no other alternatives for virucidal hand antisepsis. Long-term ingestion of ethanol in the form of alcoholic beverages can cause tumours. However, lifetime exposure to ethanol from occupational exposure textless 500~ppm does not significantly contribute to the cancer risk. Mutagenic effects were observed only at doses within the toxic range in animal studies. While reprotoxicity is linked with abuse of alcoholic beverages, there is no epidemiological evidence for this from EBHR use in healthcare facilities or from products containing ethanol in non-healthcare settings.
CONCLUSION
The body of evidence shows EBHRs have strong efficacy in killing non-enveloped viruses, whereas 1-propanol and 2-propanol do not kill non-enveloped viruses, that pose significant risk of infection. Ethanol absorbed through the skin during hand hygiene is similar to consumption of beverages with hidden ethanol content (textless 0.5% v/v), such as apple juice or kefir. There is no risk of carcinogenicity, mutagenicity or reprotoxicity from repeated use of EBHR. Hence, the WHO Task Force strongly recommend retaining ethanol as an essential constituent in hand rubs for healthcare.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The approval of ethanol by the Biocidal Products Regulation has been under evaluation since 2007. This follows concern over alcohol uptake from ethanol-based hand rubs (EBHR). If ethanol is classified as carcinogenic, mutagenic, or reprotoxic by the European Chemicals Agency (ECHA), then this would affect infection prevention and control practices.
AIM
A review was performed to prove that ethanol is toxicological uncritical and indispensable for hand antisepsis because of its unique activity against non-enveloped viruses and thus the resulting lack of alternatives. Therefore, the following main points are analyzed: The effectiveness of ethanol in hand hygiene, the evidence of ethanol at blood/tissue levels through hand hygiene in healthcare, and the evidence of toxicity of different blood/tissue ethanol levels and the non-comparability with alcoholic consumption and industrial exposure.
RESULTS
EBHR are essential for preventing infections caused by non-enveloped viruses, especially in healthcare, nursing homes, food industry and other areas. Propanols are effective against enveloped viruses as opposed to non-enveloped viruses but there are no other alternatives for virucidal hand antisepsis. Long-term ingestion of ethanol in the form of alcoholic beverages can cause tumours. However, lifetime exposure to ethanol from occupational exposure textless 500~ppm does not significantly contribute to the cancer risk. Mutagenic effects were observed only at doses within the toxic range in animal studies. While reprotoxicity is linked with abuse of alcoholic beverages, there is no epidemiological evidence for this from EBHR use in healthcare facilities or from products containing ethanol in non-healthcare settings.
CONCLUSION
The body of evidence shows EBHRs have strong efficacy in killing non-enveloped viruses, whereas 1-propanol and 2-propanol do not kill non-enveloped viruses, that pose significant risk of infection. Ethanol absorbed through the skin during hand hygiene is similar to consumption of beverages with hidden ethanol content (textless 0.5% v/v), such as apple juice or kefir. There is no risk of carcinogenicity, mutagenicity or reprotoxicity from repeated use of EBHR. Hence, the WHO Task Force strongly recommend retaining ethanol as an essential constituent in hand rubs for healthcare.
Kramer, A; Eggers, M; Exner, M; Hübner, N-O; Simon, A; Steinmann, E; Walger, P; Zwicker, P
In: GMS Hygiene and Infection Control, Bd. 17: Doc13, 2022.
@article{Kramer.2022b,
title = {Recommendation of the German Society of Hospital Hygiene (DGKH): Prevention of COVID-19 by virucidal gargling and virucidal nasal spray -- updated version April 2022},
author = {A Kramer and M Eggers and M Exner and N-O H\"{u}bner and A Simon and E Steinmann and P Walger and P Zwicker},
doi = {10.3205/dgkh000416},
year = {2022},
date = {2022-07-01},
urldate = {2022-07-01},
journal = {GMS Hygiene and Infection Control},
volume = {17: Doc13},
abstract = {The German Society of Hospital Hygiene develops guidelines, recommendations and standard operation procedures on a voluntary basis, published on the DGKH-website ([link:https://www.krankenhaushygiene.de/*https://www.krankenhaushygiene.de/]).
The original German version of this recommendation was published in April 2022 and has now been made available to the international professional public in English. Evaluating the current data on the efficacy of virucidal gargle/mouthwash solutions and nasal sprays against SARS-CoV-2 in vitro and in clinical trials, conducted with preventive or therapeutic objectives, recommendations are given for the prevention of COVID-19. The following areas are considered:
Protection of the community when regional clusters or high incidences of infection become known Protection of the community at low risk of infection Pre-exposure prophylaxis for the protection of healthcare workers Post-exposure prophylaxis
Die Deutsche Gesellschaft f\"{u}r Krankenhaushygiene (DGKH) erarbeitet Leitlinien, Empfehlungen und Standardarbeitsanweisungen auf freiwilliger Basis, die auf der DGKH-Website ver\"{o}ffentlicht werden ([link:https://www.krankenhaushygiene.de/*https://www.krankenhaushygiene.de/]).
Die deutsche Originalfassung dieser Empfehlung wurde im April 2022 ver\"{o}ffentlicht und wird jetzt auf Englisch der internationalen Fach\"{o}ffentlichkeit zur Verf\"{u}gung gestellt. In Auswertung der aktuellen Datenlage zur Wirksamkeit viruzider Gurgel-/Mundsp\"{u}ll\"{o}sungen und Nasensprays gegen SARS-CoV-2 in vitro und in klinischen Studien, die mit pr\"{a}ventiver oder therapeutischer Zielsetzung durchgef\"{u}hrt wurden, werden Empfehlungen zur Pr\"{a}vention von COVID-19 gegeben. Dabei werden folgende Bereiche ber\"{u}cksichtigt:
Schutz der Bev\"{o}lkerung bei Bekanntwerden regionaler Cluster oder hohem Infektionsgeschehen Schutz der Bev\"{o}lkerung bei geringem Infektionsrisiko Pr\"{a}expositionsprophylaxe zum Schutz des Personals im Gesundheitswesen Postexpositionsprophylaxe},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The original German version of this recommendation was published in April 2022 and has now been made available to the international professional public in English. Evaluating the current data on the efficacy of virucidal gargle/mouthwash solutions and nasal sprays against SARS-CoV-2 in vitro and in clinical trials, conducted with preventive or therapeutic objectives, recommendations are given for the prevention of COVID-19. The following areas are considered:
Protection of the community when regional clusters or high incidences of infection become known Protection of the community at low risk of infection Pre-exposure prophylaxis for the protection of healthcare workers Post-exposure prophylaxis
Die Deutsche Gesellschaft für Krankenhaushygiene (DGKH) erarbeitet Leitlinien, Empfehlungen und Standardarbeitsanweisungen auf freiwilliger Basis, die auf der DGKH-Website veröffentlicht werden ([link:https://www.krankenhaushygiene.de/*https://www.krankenhaushygiene.de/]).
Die deutsche Originalfassung dieser Empfehlung wurde im April 2022 veröffentlicht und wird jetzt auf Englisch der internationalen Fachöffentlichkeit zur Verfügung gestellt. In Auswertung der aktuellen Datenlage zur Wirksamkeit viruzider Gurgel-/Mundspüllösungen und Nasensprays gegen SARS-CoV-2 in vitro und in klinischen Studien, die mit präventiver oder therapeutischer Zielsetzung durchgeführt wurden, werden Empfehlungen zur Prävention von COVID-19 gegeben. Dabei werden folgende Bereiche berücksichtigt:
Schutz der Bevölkerung bei Bekanntwerden regionaler Cluster oder hohem Infektionsgeschehen Schutz der Bevölkerung bei geringem Infektionsrisiko Präexpositionsprophylaxe zum Schutz des Personals im Gesundheitswesen Postexpositionsprophylaxe
Eggers, M; Exner, M; Gebel, J; Rabenau, HF.; Steinmann, E; Schwebke, I
online , 2022.
@misc{Eggers.onlinevorab2022,
title = {Gemeinsame Mitteilung von VAH und der Kommission Virusdesinfektion der DVV und GfV zur Wirksamkeit von Desinfektionsmitteln bei Auftreten von akuter Hepatitis unbekannter \"{A}tiologie [non-A bis E], Stand 3.5.2022},
author = {M Eggers and M Exner and J Gebel and HF. Rabenau and E Steinmann and I Schwebke},
editor = {Gemeinsame Mitteilung Kommission Virusdesinfektion},
url = {https://www.vah-online.de},
year = {2022},
date = {2022-05-03},
urldate = {2022-05-03},
journal = {www.vah-online.de},
howpublished = {online },
keywords = {},
pubstate = {published},
tppubtype = {misc}
}
Eggers, M; Jungke, P; Wolkinger, V; Bauer, R; Kessler, U; Frank, B
Antiviral activity of plant juices and green tea against SARS-CoV-2 and influenza virus Artikel
In: Phytotherapy Research, Bd. 36, Ausg. 5, S. 2109–2115, 2022.
@article{Eggers.2022,
title = {Antiviral activity of plant juices and green tea against SARS-CoV-2 and influenza virus},
author = {M Eggers and P Jungke and V Wolkinger and R Bauer and U Kessler and B Frank},
doi = {10.1002/ptr.7431},
year = {2022},
date = {2022-03-01},
urldate = {2022-03-01},
journal = {Phytotherapy Research},
volume = {36},
issue = {5},
pages = {2109\textendash2115},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M; Baumann, A; Lilienthal, N; Steinmann, E; Steinmann, J; Hübner, NO; Rabenau, HF; Weinheimer, V; Schwebke, I
Desinfektionsmittel in der COVID-19-Pandemie: eine Herausforderung Artikel
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 65, Ausg. 1, Nr. 86–95, 2021.
@article{Eggers.2021b,
title = {Desinfektionsmittel in der COVID-19-Pandemie: eine Herausforderung},
author = {M Eggers and A Baumann and N Lilienthal and E Steinmann and J Steinmann and NO H\"{u}bner and HF Rabenau and V Weinheimer and I Schwebke},
doi = {10.1007/s00103-021-03457-z},
year = {2021},
date = {2021-11-03},
urldate = {2021-11-03},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {65},
number = {86\textendash95},
issue = {1},
abstract = {Disinfection measures have become more important as a~result of the COVID-19 pandemic in Germany. The increased need for disinfectants at the beginning of the pandemic required temporary legal regulations in order to provide a~sufficient quantity of products for the necessary disinfection in the medical sector on the one hand and for the additional demand in the population on the other. For this purpose, the Federal Institute for Drugs and Medical Devices (BfArM) and the Federal Institute for Occupational Safety and Health (BAuA) issued a general ruling, which is explained in more detail in this article. The focus was on measures for hygienic hand disinfection. However, other applications such as surface disinfection in relation to pandemic respiratory diseases are also addressed. The experience gained in ensuring the supply of disinfectants that are effective and safe to use should be used to prepare for further pandemics.
ZUSAMMENFASSUNG
Durch die COVID-19-Pandemie haben Desinfektionsma\ssnahmen auch in Deutschland an Bedeutung gewonnen. Der erh\"{o}hte Bedarf an Desinfektionsmitteln zu Beginn der Pandemie erforderte es, vor\"{u}bergehende rechtliche Regelungen zu treffen, um einerseits ausreichend Mittel f\"{u}r die notwendige Desinfektion im medizinischen Bereich und andererseits f\"{u}r den zus\"{a}tzlichen Bedarf in der Bev\"{o}lkerung zur Verf\"{u}gung zu haben. Dazu wurden vom Bundesinstitut f\"{u}r Arzneimittel und Medizinprodukte (BfArM) und der Bundesanstalt f\"{u}r Arbeitsschutz und Arbeitsmedizin (BAuA) Allgemeinverf\"{u}gungen erlassen, die in diesem Beitrag n\"{a}her erl\"{a}utert werden. Im Vordergrund stehen dabei die Ma\ssnahmen f\"{u}r die hygienische H\"{a}ndedesinfektion. Aber auch weitere Anwendungen wie die Fl\"{a}chendesinfektion im Zusammenhang mit pandemischen Atemwegserkrankungen werden er\"{o}rtert. Die Erfahrungen bei der Sicherstellung der Versorgung mit wirksamen und in der Anwendung sicheren Desinfektionsmitteln sollten f\"{u}r die Vorbereitung weiterer Pandemien genutzt werden.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
ZUSAMMENFASSUNG
Durch die COVID-19-Pandemie haben Desinfektionsmaßnahmen auch in Deutschland an Bedeutung gewonnen. Der erhöhte Bedarf an Desinfektionsmitteln zu Beginn der Pandemie erforderte es, vorübergehende rechtliche Regelungen zu treffen, um einerseits ausreichend Mittel für die notwendige Desinfektion im medizinischen Bereich und andererseits für den zusätzlichen Bedarf in der Bevölkerung zur Verfügung zu haben. Dazu wurden vom Bundesinstitut für Arzneimittel und Medizinprodukte (BfArM) und der Bundesanstalt für Arbeitsschutz und Arbeitsmedizin (BAuA) Allgemeinverfügungen erlassen, die in diesem Beitrag näher erläutert werden. Im Vordergrund stehen dabei die Maßnahmen für die hygienische Händedesinfektion. Aber auch weitere Anwendungen wie die Flächendesinfektion im Zusammenhang mit pandemischen Atemwegserkrankungen werden erörtert. Die Erfahrungen bei der Sicherstellung der Versorgung mit wirksamen und in der Anwendung sicheren Desinfektionsmitteln sollten für die Vorbereitung weiterer Pandemien genutzt werden.
Hufbauer, M; Wieland, U; Gebel, J; Steinmann, J; Akgül, B; Eggers, M
Inactivation of Polyomavirus SV40 as Surrogate for Human Papillomaviruses by Chemical Disinfectants Artikel
In: Viruses, Bd. 13, Nr. 11, S. 2207, 2021.
@article{Hufbauer.2021b,
title = {Inactivation of Polyomavirus SV40 as Surrogate for Human Papillomaviruses by Chemical Disinfectants},
author = {M Hufbauer and U Wieland and J Gebel and J Steinmann and B Akg\"{u}l and M Eggers},
doi = {10.3390/v13112207},
year = {2021},
date = {2021-11-02},
urldate = {2021-01-01},
journal = {Viruses},
volume = {13},
number = {11},
pages = {2207},
abstract = {Human papillomaviruses (HPV) are non-enveloped DNA viruses infecting cutaneous and mucosal squamous epithelia. Sexually transmitted HPV-types that are carcinogenic to humans such as HPV16 can induce cervical and other anogenital cancers. Virus transmission through fomites such as inadequately disinfected gynecological equipment is a further potential transmission route. Since HPV cannot be easily grown in cell culture, polyomavirus SV40 has been used as a surrogate virus when testing the virucidal activity of chemical disinfectants. So far, studies that have compared the virucidal activity of different disinfectants against HPV and SV40 are lacking. Here, we evaluated the susceptibility of HPV16 pseudovirus and SV40 to seven active biocidal substances using quantitative suspension tests. Ethanol, glutaraldehyde (GTA), dodecyldipropylentriamin (DPTA), and ortho-phthalaldehydes (OPA) were able to reduce the infectivity of HPV16 pseudovirus textgreater99.99% after 5 min. In contrast, isopropanol, peracetic acid (PAA), and quaternary ammonium compounds with alkylamines (QAC) only led to a slight or no reduction in infectivity. Concerning SV40, only GTA (60 min contact time), PAA, and OPA had virus-inactivating effects. In conclusion, the virucidal activity of three out of seven disinfectants tested was different for HPV16 pseudovirus and SV40. In this study, SV40 was shown to be a reliable surrogate virus for HPV when testing isopropanol-, GTA-, QAC-, and OPA-based disinfectants.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Steinhauer, K; Meister, TL; Todt, D; Krawczyk, A; Paßvogel, L; Becker, B; Paulmann, D; Bischoff, B; Eggers, M; Pfaender, S; Brill, FHH; Steinmann, E
Virucidal efficacy of different formulations for hand and surface disinfection targeting SARS CoV-2 Artikel
In: The Journal of hospital infection, Bd. 112, S. 27-30, 2021.
@article{Steinhauer.2021,
title = {Virucidal efficacy of different formulations for hand and surface disinfection targeting SARS CoV-2},
author = {K Steinhauer and TL Meister and D Todt and A Krawczyk and L Pa\ssvogel and B Becker and D Paulmann and B Bischoff and M Eggers and S Pfaender and FHH Brill and E Steinmann},
doi = {10.1016/j.jhin.2021.03.015},
year = {2021},
date = {2021-06-01},
urldate = {2021-11-02},
journal = {The Journal of hospital infection},
volume = {112},
pages = {27-30},
abstract = {In the ongoing SARS CoV-2 pandemic effective disinfection measures are needed, and guidance based on the methodological framework of the European committee for standardization (CEN) can help to choose effective disinfectants on an immediate basis. This study aimed to elucidate whether disinfectants claiming textquotedblvirucidal activity against enveloped virusestextquotedbl as specified in the European Standard EN 14476 as well as in the german national DVV/RKI guideline are effectively inactivating SARS-CoV-2. Two commercially available formulations for surface disinfection and one formulation for hand disinfection were studied regarding their virucidal activity. Based on the data of this study the enveloped SARS-CoV-2 is at least equally susceptible compared to the standard test virus vaccinia used in EN 14476 or DVV/RKI guideline. Thus, chemical disinfectants claiming textquotedblvirucidal activity against enveloped virusestextquotedbl based on EN 14476 or DVV/RKI guideline will be an effective choice to target enveloped SARS-CoV-2 as a preventive measure.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Kramer, A; Eggers, M; Hübner, N; Walger, P; Steinmann, E; Exner, M
Virucidal gargling and virucidal nasal spray Artikel
In: GMS Hygiene and Infection Control, Bd. 16, Nr. Doc02, 2021.
@article{Kramer.2021,
title = {Virucidal gargling and virucidal nasal spray},
author = {A Kramer and M Eggers and N H\"{u}bner and P Walger and E Steinmann and M Exner},
doi = {10.3205/DGKH000373},
year = {2021},
date = {2021-01-01},
journal = {GMS Hygiene and Infection Control},
volume = {16},
number = {Doc02},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M; Schwebke, I; Suchomel, M; Fotheringham, V; Gebel, J; Meyer, B; Morace, G; Roedger, HJ; Roques, C; Visa, P; Steinhauer, K
In: Euro Surveillance, Bd. 26, Nr. 3, S. pii=2000708., 2021.
@article{Eggers.2021,
title = {The European tiered approach for virucidal efficacy testing - rationale for rapidly selecting disinfectants against emerging and re-emerging viral diseases},
author = {M Eggers and I Schwebke and M Suchomel and V Fotheringham and J Gebel and B Meyer and G Morace and HJ Roedger and C Roques and P Visa and K Steinhauer},
doi = {10.2807/1560-7917.ES.2021.26.3.2000708},
year = {2021},
date = {2021-01-01},
journal = {Euro Surveillance},
volume = {26},
number = {3},
pages = {pii=2000708.},
abstract = {When facing an emerging virus outbreak such as severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), a quick reaction time is key to control the spread. It takes time to develop antivirals and vaccines, and implement vaccination campaigns. Therefore, preventive measures such as rapid isolation of cases and identification and early quarantine of cases' close contacts-as well as masks, physical distancing, hand hygiene, surface disinfection and air control-are crucial to reduce the risk of transmission. In this context, disinfectants and antiseptics with proven efficacy against the outbreak virus should be used. However, biocidal formulations are quite complex and may include auxiliary substances such as surfactants or emollients in addition to active substances. In order to evaluate disinfectants' efficacy objectively, meaningful efficacy data are needed. Therefore, the European Committee for Standardisation technical committee 216 'Chemical disinfectants and antiseptics' Working Group 1 (medical area) has developed standards for efficacy testing. The European tiered approach grades the virucidal efficacy in three levels, with corresponding marker test viruses. In the case of SARS-CoV-2, disinfectants with proven activity against vaccinia virus, the marker virus for the European claim 'active against enveloped viruses', should be used to ensure effective hygiene procedures to control the pandemic.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Stathis, C; Victoria, N; Loomis, K; Nguyen, SA; Eggers, M; Septimus, E; Safdar, N
Review of the use of nasal and oral antiseptics during a global pandemic Artikel
In: Future microbiology, Bd. 16, Nr. 2, S. 119-130, 2021.
@article{Stathis.2021,
title = {Review of the use of nasal and oral antiseptics during a global pandemic},
author = {C Stathis and N Victoria and K Loomis and SA Nguyen and M Eggers and E Septimus and N Safdar},
doi = {10.2217/fmb-2020-0286},
year = {2021},
date = {2021-01-01},
journal = {Future microbiology},
volume = {16},
number = {2},
pages = {119-130},
abstract = {A review of nasal sprays and gargles with antiviral properties suggests that a number of commonly used antiseptics including povidone-iodine, ListerinecircledR, iota-carrageenan~and chlorhexidine should be studied in clinical trials to mitigate both the progression and transmission of SARS-CoV-2. Several of these antiseptics have demonstrated the ability to cut the viral load of SARS-CoV-2 by 3-4 log10 in 15-30 s~in vitro. In addition, hypertonic saline targets viral replication by increasing hypochlorous acid inside the cell. A number of clinical trials are in process to study these interventions both for prevention of transmission, prophylaxis after exposure, and to diminish progression by reduction of viral load in the early stages of infection.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Hufbauer, M; Wieland, U; Gebel, J; Steinmann, J; Akgül, B; Eggers, M
Inactivation of Polyomavirus SV40 as Surrogate for Human Papillomaviruses by Chemical Disinfectants Artikel
In: Viruses, Bd. 13, Nr. 11, S. 2207, 2021.
@article{Hufbauer.2021,
title = {Inactivation of Polyomavirus SV40 as Surrogate for Human Papillomaviruses by Chemical Disinfectants},
author = {M Hufbauer and U Wieland and J Gebel and J Steinmann and B Akg\"{u}l and M Eggers},
doi = {10.3390/v13112207},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Viruses},
volume = {13},
number = {11},
pages = {2207},
abstract = {Human papillomaviruses (HPV) are non-enveloped DNA viruses infecting cutaneous and mucosal squamous epithelia. Sexually transmitted HPV-types that are carcinogenic to humans such as HPV16 can induce cervical and other anogenital cancers. Virus transmission through fomites such as inadequately disinfected gynecological equipment is a further potential transmission route. Since HPV cannot be easily grown in cell culture, polyomavirus SV40 has been used as a surrogate virus when testing the virucidal activity of chemical disinfectants. So far, studies that have compared the virucidal activity of different disinfectants against HPV and SV40 are lacking. Here, we evaluated the susceptibility of HPV16 pseudovirus and SV40 to seven active biocidal substances using quantitative suspension tests. Ethanol, glutaraldehyde (GTA), dodecyldipropylentriamin (DPTA), and ortho-phthalaldehydes (OPA) were able to reduce the infectivity of HPV16 pseudovirus textgreater99.99% after 5 min. In contrast, isopropanol, peracetic acid (PAA), and quaternary ammonium compounds with alkylamines (QAC) only led to a slight or no reduction in infectivity. Concerning SV40, only GTA (60 min contact time), PAA, and OPA had virus-inactivating effects. In conclusion, the virucidal activity of three out of seven disinfectants tested was different for HPV16 pseudovirus and SV40. In this study, SV40 was shown to be a reliable surrogate virus for HPV when testing isopropanol-, GTA-, QAC-, and OPA-based disinfectants.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M; Benzinger, C; Suchomel, M; Hjorth, E
In: Future microbiology, Bd. 15, S. 1335-1341, 2020.
@article{Eggers.2020c,
title = {Virucidal activity of three ethanol-based handrubs against murine norovirus in a hand hygiene clinical simulation study},
author = {M Eggers and C Benzinger and M Suchomel and E Hjorth},
doi = {10.2217/fmb-2020-0168},
year = {2020},
date = {2020-09-01},
journal = {Future microbiology},
volume = {15},
pages = {1335-1341},
abstract = {Aim: We evaluated the efficacy of three ethanol-based handrubs against murine norovirus~in a proposed clinical simulation test (prEN 17430). Materials \& methods: Virucidal activity was determined in 18 volunteers using three~handrubs: ethanol 72.4~and 89.5% v/v solutions, and 86% v/v gel. Subjects underwent testing with each product (3/6~ml~for 15/30~s) and a reference solution (6~ml~70% v/v ethanol for 60~s). Results: Against murine norovirus, the reduction factors~(RF;~RF mean~$pm$ standard deviation~log10 reduction of postsampling) for ethanol gel 86% v/v (RF 1.96~$pm$~0.64), ethanol 89.5% v/v (RF 2.49~$pm$~0.59)~and ethanol 72.4% v/v (RF 2.61~$pm$~0.50), were all significantly superior to that of~the reference solution. Conclusion: All three handrubs passed the criteria set out in prEN 17430 and exhibited excellent virucidal efficacy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rabenau, HF; Schwebke, I; Blümel, J; Eggers, M; Glebe, D; Rapp, I; Sauerbrei, A; Steinmann, E; Steinmann, J; Willkommen, He; Wutzler, P
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 63, Nr. 5, S. 645–655, 2020.
@article{Rabenau.2020,
title = {Guideline for testing chemical disinfectants regarding their virucidal activity within the field of human medicine : as of December 1st, 2014 Prepared by the German Association for the Control of Virus Diseases (DVV) and the Robert Koch Institute (RKI)},
author = {HF Rabenau and I Schwebke and J Bl\"{u}mel and M Eggers and D Glebe and I Rapp and A Sauerbrei and E Steinmann and J Steinmann and He Willkommen and P Wutzler},
doi = {10.1007/s00103-020-03115-w},
year = {2020},
date = {2020-01-01},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {63},
number = {5},
pages = {645--655},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Hübner, N-O; Eggers, M; Schwebke, I; Suchomel, M
Händedesinfektion unter den Bedingungen der SARSCoV- 2-Pandemie Artikel
In: Epidemiologisches Bulletin, Bd. 19, S. 13-19, 2020.
@article{Hubner.2020,
title = {H\"{a}ndedesinfektion unter den Bedingungen der SARSCoV- 2-Pandemie},
author = {N-O H\"{u}bner and M Eggers and I Schwebke and M Suchomel},
url = {https://edoc.rki.de/handle/176904/6774?show=full},
doi = {10.25646/6861},
year = {2020},
date = {2020-01-01},
journal = {Epidemiologisches Bulletin},
volume = {19},
pages = {13-19},
abstract = {Die aktuelle SARS-CoV-2-Pandemie f\"{u}hrt uns zum einen den Stellenwert der H\"{a}ndedesinfektion zum Schutz der Patienten und Besch\"{a}ftigten vor Augen. Zum anderen zeigt sie, wie wichtig die stete Verf\"{u}gbarkeit von H\"{a}ndedesinfektionsmitteln ist, deren Wirksamkeit, Qualit\"{a}t und Unbedenklichkeit nachgewiesen und die unter praktischen Bedingungen tauglich sind. Aussagen u. a. zu Bestandteilen, zur Deklaration der Wirksamkeit und zur Qualit\"{a}t von alkoholischen H\"{a}ndedesinfektionsmitteln werden im Epidemiologischen Bulletin 19/2020 n\"{a}her ausgef\"{u}hrt.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Anderson, DE; Sivalingam, V; Kang, A Eng Zheng; Ananthanarayanan, A; Arumugam, H; Jenkins, TM; Hadjiat, Y; Eggers, M
In: Infectious diseases and therapy, Bd. 9, Nr. 3, S. 669–675, 2020.
@article{Anderson.2020,
title = {Povidone-Iodine Demonstrates Rapid In Vitro Virucidal Activity Against SARS-CoV-2, The Virus Causing COVID-19 Disease},
author = {DE Anderson and V Sivalingam and A Eng Zheng Kang and A Ananthanarayanan and H Arumugam and TM Jenkins and Y Hadjiat and M Eggers},
doi = {10.1007/s40121-020-00316-3},
year = {2020},
date = {2020-01-01},
journal = {Infectious diseases and therapy},
volume = {9},
number = {3},
pages = {669\textendash675},
abstract = {INTRODUCTION
As of 22 June 2020, Severe Acute Respiratory Syndrome (SARS)-coronavirus (CoV)-2 has infected more than 8.95 million people worldwide, causing textgreater 468,000 deaths. The virus is transmitted through respiratory droplets and physical contact from contaminated surfaces to the mucosa. Hand hygiene and oral decontamination among other measures are key to preventing the spread of the virus. We report the in vitro virucidal activity of topical and oral povidone-iodine (PVP-I) products against SARS-CoV-2.
METHODS
Suspension assays were used to assess the virucidal activity of PVP-I against SARS-CoV-2. Products were tested at a contact time of 30~s for virucidal activity. Viral titres were calculated using the Spearman-K\"{a}rber method and reported as median tissue culture infectious dose (TCID50)/mL.
RESULTS
All four products [antiseptic solution (PVP-I 10%), skin cleanser (PVP-I 7.5%), gargle and mouth wash (PVP-I 1%) and throat spray (PVP-I 0.45%)] achieved $geq$ 99.99% virucidal activity against SARS-CoV-2, corresponding to $geq$ 4 log10 reduction of virus titre, within 30~s of contact.
CONCLUSION
This study provides evidence of rapid and effective virucidal activity of PVP-I against SARS-CoV-2. PVP-I-based products are widely available for medical and personal use for hand hygiene and oral decontamination, and could be readily integrated into coronavirus disease, COVID-19, infection control measures in hospital and community settings.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
As of 22 June 2020, Severe Acute Respiratory Syndrome (SARS)-coronavirus (CoV)-2 has infected more than 8.95 million people worldwide, causing textgreater 468,000 deaths. The virus is transmitted through respiratory droplets and physical contact from contaminated surfaces to the mucosa. Hand hygiene and oral decontamination among other measures are key to preventing the spread of the virus. We report the in vitro virucidal activity of topical and oral povidone-iodine (PVP-I) products against SARS-CoV-2.
METHODS
Suspension assays were used to assess the virucidal activity of PVP-I against SARS-CoV-2. Products were tested at a contact time of 30~s for virucidal activity. Viral titres were calculated using the Spearman-Kärber method and reported as median tissue culture infectious dose (TCID50)/mL.
RESULTS
All four products [antiseptic solution (PVP-I 10%), skin cleanser (PVP-I 7.5%), gargle and mouth wash (PVP-I 1%) and throat spray (PVP-I 0.45%)] achieved $geq$ 99.99% virucidal activity against SARS-CoV-2, corresponding to $geq$ 4 log10 reduction of virus titre, within 30~s of contact.
CONCLUSION
This study provides evidence of rapid and effective virucidal activity of PVP-I against SARS-CoV-2. PVP-I-based products are widely available for medical and personal use for hand hygiene and oral decontamination, and could be readily integrated into coronavirus disease, COVID-19, infection control measures in hospital and community settings.
Boursillon, D; Kocics, J; Eggers, M; Elts, B
Reiniger mit Zusatz von Mikroorganismen (MARP) Artikel
In: Hygiene + Medizin, Bd. 45, Nr. 7/8, S. 98–101, 2020.
@article{Boursillon.2020,
title = {Reiniger mit Zusatz von Mikroorganismen (MARP)},
author = {D Boursillon and J Kocics and M Eggers and B Elts},
year = {2020},
date = {2020-01-01},
journal = {Hygiene + Medizin},
volume = {45},
number = {7/8},
pages = {98--101},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M; Hitz, DA; Elts, B
Reinigung von Oberflächen - Eine mikrobiologische Untersuchung und Bewertung der Reinigung in öffentlichen Bereichen Artikel
In: Hygiene + Medizin, Bd. 45, Nr. 7-8, S. D102–D106, 2020.
@article{Eggers.2020,
title = {Reinigung von Oberfl\"{a}chen - Eine mikrobiologische Untersuchung und Bewertung der Reinigung in \"{o}ffentlichen Bereichen},
author = {M Eggers and DA Hitz and B Elts},
year = {2020},
date = {2020-01-01},
journal = {Hygiene + Medizin},
volume = {45},
number = {7-8},
pages = {D102--D106},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M; Rabenau, H; Blümel, J; Fickenscher, H; Geisel, B; Glebe, D; Hengel, H; Marschang, R; Reiche, S; Steinmann, E; Steinmann, J; Schwebke, I
In: Epidemiologisches Bulletin, Nr. 36, S. 3–14, 2020.
@article{Eggers.2020b,
title = {Einsatz geeigneter Desinfektionsmitteln bei gentechnisch ver\"{a}nderten Viren und viralen Vektoren -- Stellungnahme der Kommission f\"{u}r Virusdesinfektion der Deutschen Vereinigung zur Bek\"{a}mpfung der Viruskrankheiten (DVV) e. V. und der Gesellschaft f\"{u}r Virologie (GfV) e. V},
author = {M Eggers and H Rabenau and J Bl\"{u}mel and H Fickenscher and B Geisel and D Glebe and H Hengel and R Marschang and S Reiche and E Steinmann and J Steinmann and I Schwebke},
doi = {10.25646/7030},
year = {2020},
date = {2020-01-01},
journal = {Epidemiologisches Bulletin},
number = {36},
pages = {3--14},
abstract = {Im Epidemiologischen Bulletin 36/2020 haben Mitglieder der Kommission f\"{u}r Virusdesinfektion der DVV/GfV eine \"{U}bersicht h\"{a}ufig verwendeter gentechnisch ver\"{a}nderter Organismen zusammengestellt, davon ausgehend werden Aspekte der Auswahl und Anwendung von Desinfektionsmitteln n\"{a}her er\"{o}rtert. Dabei wurden neben Vertretern von Bundesoberbeh\"{o}rden, wie des FLI, des PEI und des RKI, auch Vertreter des \"{O}GD und virologischer Institute aus Universit\"{a}ten einbezogen.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Elts, B; Rager, A-M; Boursillon, D; Eggers, M
Aufbereitung von Reinigungstextilien in der Krankenhausreinigung Artikel
In: Hygiene + Medizin, Bd. 45, Nr. 7/8, S. D80–D89, 2020.
@article{Elts.2020,
title = {Aufbereitung von Reinigungstextilien in der Krankenhausreinigung},
author = {B Elts and A-M Rager and D Boursillon and M Eggers},
year = {2020},
date = {2020-01-01},
journal = {Hygiene + Medizin},
volume = {45},
number = {7/8},
pages = {D80--D89},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Kramer, A; Eggers, M
Prävention respiratorischer Virusinfektionen durch viruzide Schleimhautantiseptik bei medizinischem Personal und in der Bevölkerung Artikel
In: Hygiene + Medizin, Bd. 45, Nr. 9, S. D118–D124, 2020.
@article{Kramer.2020,
title = {Pr\"{a}vention respiratorischer Virusinfektionen durch viruzide Schleimhautantiseptik bei medizinischem Personal und in der Bev\"{o}lkerung},
author = {A Kramer and M Eggers},
year = {2020},
date = {2020-01-01},
journal = {Hygiene + Medizin},
volume = {45},
number = {9},
pages = {D118\textendashD124},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rabenau, HF; Schwebke, I; Blümel, J; Eggers, M; Rapp, I; Steinmann, J; Willkommen, H
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 63, Nr. 5, S. 657–659, 2020.
@article{Rabenau.2020b,
title = {Comment on the significance, application and determination of the large volume plating (LVP) 2. Communication of the DVV/GfV Virus Disinfection Expert Committee on the DVV/RKI Guideline in the version of December 1st, 2014},
author = {HF Rabenau and I Schwebke and J Bl\"{u}mel and M Eggers and I Rapp and J Steinmann and H Willkommen},
doi = {10.1007/s00103-020-03117-8},
year = {2020},
date = {2020-01-01},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {63},
number = {5},
pages = {657--659},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rager, A; Eggers, M; Eilts, B; Klingshirn, A
In: Hauswirtschaft und Wissenschaft, Bd. 68, S. 1, 2020.
@article{Rager.2020,
title = {Entwicklung eines neuen Bioindikatorsystems zur Pr\"{u}fung der Hygienewirkung von Geschirrsp\"{u}lverfahren unter besonderer Ber\"{u}cksichtigung von englumigem Sp\"{u}lgut},
author = {A Rager and M Eggers and B Eilts and A Klingshirn},
doi = {10.23782/HUWtextunderscore 10textunderscore 2020},
year = {2020},
date = {2020-01-01},
journal = {Hauswirtschaft und Wissenschaft},
volume = {68},
pages = {1},
abstract = {Die Aufbereitung von Sp\"{u}lgut aus hygienisch sensiblen Bereichen stellt Einrichtungen wie z. B. Kindertagesst\"{a}tten und Pflegeheime vor Herausforderungen. In Einrichtungen, in denen Menschen mit noch nicht vollst\"{a}ndig ausgebildeter oder eingeschr\"{a}nkter Immunabwehr untergebracht sind, ist die Gew\"{a}hrleistung von hygienisch einwandfreiem Sp\"{u}lgut zur Vermeidung der \"{U}bertragung von Krankheitserregern sicherzustellen. Der Einsatz von Haushaltsgeschirrsp\"{u}lern in hygienisch sensiblen Bereichen wird von \"{U}berwachungsbeh\"{o}rden daher kritisch betrachtet. Mittels eines neuentwickelten Bioindikatorsystems soll die hygienische Aufbereitung von v. a. englumigem Sp\"{u}lgut in Geschirrsp\"{u}lmaschinen in diesen Einrichtungen untersucht werden.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Gemein, S; Gebel, J; Christiansen, B; Martiny, H; Vossebein, L; Brill, F H H; Decius, M; Eggers, M; Koburger-Janssen, T; Meckel, M; Werner, S; Hunsinger, B; Selhorst, T; Kampf, G; Exner, M
In: The Journal of hospital infection, Bd. 103, Nr. 1, S. 78–84, 2019.
@article{Gemein.2019,
title = {Interlaboratory reproducibility of a test method following 4-field test methodology to evaluate the susceptibility of Clostridium difficile spores},
author = {S Gemein and J Gebel and B Christiansen and H Martiny and L Vossebein and F H H Brill and M Decius and M Eggers and T Koburger-Janssen and M Meckel and S Werner and B Hunsinger and T Selhorst and G Kampf and M Exner},
doi = {10.1016/j.jhin.2019.04.011},
year = {2019},
date = {2019-01-01},
journal = {The Journal of hospital infection},
volume = {103},
number = {1},
pages = {78--84},
abstract = {BACKGROUND
Sporicidal surface disinfection is recommended to control transmission of Clostridium difficile in healthcare facilities. EN 17126 provides a method to determine the sporicidal activity in suspension and has been approved as a European standard. In addition, a sporicidal surface test has been proposed.
AIM
To determine the interlaboratory reproducibility of a test method for evaluating the susceptibility of a C.~difficile spore preparation to a biocidal formulation following the 4-field test (EN 16615 methodology).
METHODS
Nine laboratories participated. C.~difficile NCTC 13366 spores were used. Glutaraldehyde (1% and 6%; 15 min) and peracetic acid (PAA; 0.01% and 0.04%; 15 min) were used to determine the spores' susceptibility in suspension in triplicate.
FINDINGS
One-percent glutaraldehyde revealed a mean decimal log10 reduction of 1.03 with variable results in the nine laboratories (0.37-1.49) and a reproducibility of 0.38. The effect of 6% glutaraldehyde was stronger (mean: 2.05; range: 0.96-4.29; reproducibility: 0.86). PAA revealed similar results. An exemplary biocidal formulation based on 5% PAA was used at 0.5% (non-effective concentration) and 4% (effective concentration) to determine the sporicidal efficacy (4-field test) under clean conditions in triplicate with a contact time of 15 min. When used at 0.5% it demonstrated an overall log10 reduction of 2.68 (range: 2.35-3.57) and at 4% of 4.61 (range: 3.82-5.71). The residual contamination on the three primarily uncontaminated test fields was textless50 cfu/25 cm2 in one out of nine laboratories (0.5%) and in seven out of nine laboratories (4%).
CONCLUSION
The interlaboratory reproducibility seems to be robust.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Sporicidal surface disinfection is recommended to control transmission of Clostridium difficile in healthcare facilities. EN 17126 provides a method to determine the sporicidal activity in suspension and has been approved as a European standard. In addition, a sporicidal surface test has been proposed.
AIM
To determine the interlaboratory reproducibility of a test method for evaluating the susceptibility of a C.~difficile spore preparation to a biocidal formulation following the 4-field test (EN 16615 methodology).
METHODS
Nine laboratories participated. C.~difficile NCTC 13366 spores were used. Glutaraldehyde (1% and 6%; 15 min) and peracetic acid (PAA; 0.01% and 0.04%; 15 min) were used to determine the spores' susceptibility in suspension in triplicate.
FINDINGS
One-percent glutaraldehyde revealed a mean decimal log10 reduction of 1.03 with variable results in the nine laboratories (0.37-1.49) and a reproducibility of 0.38. The effect of 6% glutaraldehyde was stronger (mean: 2.05; range: 0.96-4.29; reproducibility: 0.86). PAA revealed similar results. An exemplary biocidal formulation based on 5% PAA was used at 0.5% (non-effective concentration) and 4% (effective concentration) to determine the sporicidal efficacy (4-field test) under clean conditions in triplicate with a contact time of 15 min. When used at 0.5% it demonstrated an overall log10 reduction of 2.68 (range: 2.35-3.57) and at 4% of 4.61 (range: 3.82-5.71). The residual contamination on the three primarily uncontaminated test fields was textless50 cfu/25 cm2 in one out of nine laboratories (0.5%) and in seven out of nine laboratories (4%).
CONCLUSION
The interlaboratory reproducibility seems to be robust.
Eggers, M; Koburger-Janssen, T; Eickmann, M; Zorn, J
In: Infectious diseases and therapy, Bd. 7, Nr. 2, S. 249–259, 2018, ISSN: 2193-8229.
@article{Eggers.2018,
title = {In Vitro Bactericidal and Virucidal Efficacy of Povidone-Iodine Gargle/Mouthwash Against Respiratory and Oral Tract Pathogens},
author = {M Eggers and T Koburger-Janssen and M Eickmann and J Zorn},
doi = {10.1007/s40121-018-0200-7},
issn = {2193-8229},
year = {2018},
date = {2018-01-01},
journal = {Infectious diseases and therapy},
volume = {7},
number = {2},
pages = {249--259},
abstract = {INTRODUCTION
Recent virus epidemics and rising antibiotic resistance highlight the importance of hygiene measures to prevent and control outbreaks. We investigated the in vitro bactericidal and virucidal efficacy of povidone-iodine (PVP-I) 7% gargle/mouthwash at defined dilution against oral and respiratory tract pathogens.
METHODS
PVP-I was tested against Klebsiella pneumoniae and Streptococcus pneumoniae according to bactericidal quantitative suspension test EN13727 and against severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses (SARS-CoV and MERS-CoV), rotavirus strain Wa and influenza virus A subtype H1N1 according to virucidal quantitative suspension test EN14476. PVP-I 7% gargle/mouthwash was diluted 1:30 with water to a concentration of 0.23% (the recommended concentration for textquotedblreal-lifetextquotedbl use in Japan) and tested at room temperature under clean conditions [0.3~g/l bovine serum albumin (BSA), viruses only] and dirty conditions (3.0~g/l BSA + 3.0~ml/l erythrocytes) as an interfering substance for defined contact times (minimum 15~s). Rotavirus was tested without protein load. A $geq$ 5 log10 (99.999%) decrease of bacteria and $geq$ 4 log10 (99.99%) reduction in viral titre represented effective bactericidal and virucidal activity, respectively, per European standards.
RESULTS
PVP-I gargle/mouthwash diluted 1:30 (equivalent to a concentration of 0.23% PVP-I) showed effective bactericidal activity against Klebsiella pneumoniae and Streptococcus pneumoniae and rapidly inactivated SARS-CoV, MERS-CoV, influenza virus A (H1N1) and rotavirus after 15~s of exposure.
CONCLUSION
PVP-I 7% gargle/mouthwash showed rapid bactericidal activity and virucidal efficacy in vitro at a concentration of 0.23% PVP-I and may provide a protective oropharyngeal hygiene measure for individuals at high risk of exposure to oral and respiratory pathogens.
FUNDING
Mundipharma Research GmbH \& Co. KG (MRG).},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Recent virus epidemics and rising antibiotic resistance highlight the importance of hygiene measures to prevent and control outbreaks. We investigated the in vitro bactericidal and virucidal efficacy of povidone-iodine (PVP-I) 7% gargle/mouthwash at defined dilution against oral and respiratory tract pathogens.
METHODS
PVP-I was tested against Klebsiella pneumoniae and Streptococcus pneumoniae according to bactericidal quantitative suspension test EN13727 and against severe acute respiratory syndrome and Middle East respiratory syndrome coronaviruses (SARS-CoV and MERS-CoV), rotavirus strain Wa and influenza virus A subtype H1N1 according to virucidal quantitative suspension test EN14476. PVP-I 7% gargle/mouthwash was diluted 1:30 with water to a concentration of 0.23% (the recommended concentration for textquotedblreal-lifetextquotedbl use in Japan) and tested at room temperature under clean conditions [0.3~g/l bovine serum albumin (BSA), viruses only] and dirty conditions (3.0~g/l BSA + 3.0~ml/l erythrocytes) as an interfering substance for defined contact times (minimum 15~s). Rotavirus was tested without protein load. A $geq$ 5 log10 (99.999%) decrease of bacteria and $geq$ 4 log10 (99.99%) reduction in viral titre represented effective bactericidal and virucidal activity, respectively, per European standards.
RESULTS
PVP-I gargle/mouthwash diluted 1:30 (equivalent to a concentration of 0.23% PVP-I) showed effective bactericidal activity against Klebsiella pneumoniae and Streptococcus pneumoniae and rapidly inactivated SARS-CoV, MERS-CoV, influenza virus A (H1N1) and rotavirus after 15~s of exposure.
CONCLUSION
PVP-I 7% gargle/mouthwash showed rapid bactericidal activity and virucidal efficacy in vitro at a concentration of 0.23% PVP-I and may provide a protective oropharyngeal hygiene measure for individuals at high risk of exposure to oral and respiratory pathogens.
FUNDING
Mundipharma Research GmbH & Co. KG (MRG).
Eggers, M; Koburger-Janssen, T; Ward, L S; Newby, C; Müller, S
In: Infectious diseases and therapy, Bd. 7, Nr. 2, S. 235–247, 2018, ISSN: 2193-8229.
@article{Eggers.2018b,
title = {Bactericidal and Virucidal Activity of Povidone-Iodine and Chlorhexidine Gluconate Cleansers in an In Vivo Hand Hygiene Clinical Simulation Study},
author = {M Eggers and T Koburger-Janssen and L S Ward and C Newby and S M\"{u}ller},
doi = {10.1007/s40121-018-0202-5},
issn = {2193-8229},
year = {2018},
date = {2018-01-01},
journal = {Infectious diseases and therapy},
volume = {7},
number = {2},
pages = {235--247},
abstract = {INTRODUCTION
Standard in vitro and in vivo tests help demonstrate efficacy of hand hygiene products; however, there is no standard in vivo test method for viruses. We investigated the bactericidal and virucidal efficacy of povidone-iodine (PVP-I) 7.5% scalp and skin cleanser, chlorhexidine gluconate (CHG) 4% hand cleanser and the reference hand wash (soft soap) in 15 healthy volunteers following European Standard EN1499 (hygienic hand wash test method for bacteria), which was adapted for virucidal testing.
METHODS
Separate test series were performed for bactericidal (Escherichia coli) and virucidal [murine norovirus (MNV)] testing. After pre-washing and artificial contamination of hands with test organisms, volunteers underwent testing with 3 and 5~mL of each product for contact times of 15, 30 and 60~s according to a Latin-square randomization. The number of test organisms released from fingertips into sampling fluids was assessed before and after hand washing and mean log10 reduction factor (RF) was calculated. RFs (test-reference) were compared using a Wilcoxon-Wilcox multiple comparisons test per EN1499; efficacy was concluded if p $\leq$ 0.01.
RESULTS
PVP-I 7.5% and CHG 4% cleansers both passed EN1499 requirements against E. coli, with statistically significantly greater (p $\leq$ 0.01) mean log10 RFs compared with reference soft soap across all tests (PVP-I: 4.09-5.27; CHG: 4.12-5.22; soap: 2.75-3.11). The experimental design using EN1499 was applicable to testing with MNV as discriminatory and reproducible results were generated. Mean log10 RFs of MNV were statistically significantly greater for PVP-I (1.57-2.57) compared with soft soap (1.24-1.62), while mean log10 RFs with CHG (0.90-1.34) were lower than for soft soap across all tests.
CONCLUSION
PVP-I 7.5% cleanser showed superior efficacy against MNV compared to soft soap and CHG 4% cleanser, while both PVP-I and CHG were superior to soft soap against E. coli. The experimental set-up may be applicable to future testing for antiviral hand washes.
FUNDING
Mundipharma Manufacturing Pte Ltd. Plain language summary available for this article.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Standard in vitro and in vivo tests help demonstrate efficacy of hand hygiene products; however, there is no standard in vivo test method for viruses. We investigated the bactericidal and virucidal efficacy of povidone-iodine (PVP-I) 7.5% scalp and skin cleanser, chlorhexidine gluconate (CHG) 4% hand cleanser and the reference hand wash (soft soap) in 15 healthy volunteers following European Standard EN1499 (hygienic hand wash test method for bacteria), which was adapted for virucidal testing.
METHODS
Separate test series were performed for bactericidal (Escherichia coli) and virucidal [murine norovirus (MNV)] testing. After pre-washing and artificial contamination of hands with test organisms, volunteers underwent testing with 3 and 5~mL of each product for contact times of 15, 30 and 60~s according to a Latin-square randomization. The number of test organisms released from fingertips into sampling fluids was assessed before and after hand washing and mean log10 reduction factor (RF) was calculated. RFs (test-reference) were compared using a Wilcoxon-Wilcox multiple comparisons test per EN1499; efficacy was concluded if p $łeq$ 0.01.
RESULTS
PVP-I 7.5% and CHG 4% cleansers both passed EN1499 requirements against E. coli, with statistically significantly greater (p $łeq$ 0.01) mean log10 RFs compared with reference soft soap across all tests (PVP-I: 4.09-5.27; CHG: 4.12-5.22; soap: 2.75-3.11). The experimental design using EN1499 was applicable to testing with MNV as discriminatory and reproducible results were generated. Mean log10 RFs of MNV were statistically significantly greater for PVP-I (1.57-2.57) compared with soft soap (1.24-1.62), while mean log10 RFs with CHG (0.90-1.34) were lower than for soft soap across all tests.
CONCLUSION
PVP-I 7.5% cleanser showed superior efficacy against MNV compared to soft soap and CHG 4% cleanser, while both PVP-I and CHG were superior to soft soap against E. coli. The experimental set-up may be applicable to future testing for antiviral hand washes.
FUNDING
Mundipharma Manufacturing Pte Ltd. Plain language summary available for this article.
Schwebke, I; Eggers, M; Gebel, J; Geisel, B; Glebe, D; Rapp, I; Steinmann, J; Rabenau, H F
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 60, Nr. 3, S. 353–363, 2017, ISSN: 1436-9990.
@article{Schwebke.2017,
title = {Pr\"{u}fung und Deklaration der Wirksamkeit von Desinfektionsmitteln gegen Viren zur Anwendung im human-medizinischen Bereich : Stellungnahme des Arbeitskreises Viruzidie beim Robert Koch-Institut},
author = {I Schwebke and M Eggers and J Gebel and B Geisel and D Glebe and I Rapp and J Steinmann and H F Rabenau},
doi = {10.1007/s00103-016-2509-2},
issn = {1436-9990},
year = {2017},
date = {2017-01-01},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {60},
number = {3},
pages = {353--363},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Schwebke, I; Eggers, M; Gebel, J; Geisel, B; Steinmann, J; Hübner, N-O; Rapp, I; Rabenau, H F
In: Epidemiologisches Bulletin, Nr. 18/19, S. 171–172, 2017.
@article{Schwebke.2017b,
title = {H\"{a}ndedesinfektionsmittel: Welche Bedeutung und Konsequenzen hat der neue Wirkbereich „begrenzt viruzid PLUS“?},
author = {I Schwebke and M Eggers and J Gebel and B Geisel and J Steinmann and N-O H\"{u}bner and I Rapp and H F Rabenau},
doi = {10.17886/EpiBull-2017-026},
year = {2017},
date = {2017-01-01},
journal = {Epidemiologisches Bulletin},
number = {18/19},
pages = {171--172},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rabenau, H F; Schwebke, I; Blümel, J; Eggers, M; Rapp, I; Steinmann, J; Willkommen, H
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 59, Nr. 4, S. 540–542, 2016, ISSN: 1436-9990.
@article{Rabenau.2016,
title = {2. Mitteilung des DVV/GfV-Fachausschusses Virusdesinfektion zur DVV/RKI-Leitlinie in der Fassung vom 01.12.2014 : Erl\"{a}uterung zur Bedeutung, Anwendung und Berechnung des textquotedblLarge-Volume-Platingstextquotedbl (LVP)},
author = {H F Rabenau and I Schwebke and J Bl\"{u}mel and M Eggers and I Rapp and J Steinmann and H Willkommen},
doi = {10.1007/s00103-016-2325-8},
issn = {1436-9990},
year = {2016},
date = {2016-01-01},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {59},
number = {4},
pages = {540--542},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M
Viruzidie 4.0 Artikel
In: Der Mikrobiologe, Bd. 26, Nr. 3, S. 122, 2016, ISSN: 0943-674X.
@article{Eggers.2016,
title = {Viruzidie 4.0},
author = {M Eggers },
url = {http://www.zbmed.de/ccmedimages/2016/ZBMED-2016101851511-17.pdf},
issn = {0943-674X},
year = {2016},
date = {2016-01-01},
journal = {Der Mikrobiologe},
volume = {26},
number = {3},
pages = {122},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Rabenau, H F; Schwebke, I; Blümel, J; Eggers, Maren; Glebe, D; Rapp, I; Sauerbrei, A; Steinmann, E; Steinmann, J; Willkommen, H; Wutzler, P
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 58, Nr. 4-5, S. 493–504, 2015, ISSN: 1436-9990.
@article{Rabenau.2015,
title = {Leitlinie der Deutschen Vereinigung zur Bek\"{a}mpfung der Viruskrankheiten (DVV) e. V. und des Robert Koch-Instituts (RKI) zur Pr\"{u}fung von chemischen Desinfektionsmitteln auf Wirksamkeit gegen Viren in der Humanmedizin : Fassung vom 1. Dezember 2014},
author = {H F Rabenau and I Schwebke and J Bl\"{u}mel and Maren Eggers and D Glebe and I Rapp and A Sauerbrei and E Steinmann and J Steinmann and H Willkommen and P Wutzler},
doi = {10.1007/s00103-015-2131-8},
issn = {1436-9990},
year = {2015},
date = {2015-01-01},
urldate = {2015-01-01},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {58},
number = {4-5},
pages = {493--504},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Schwebke, I; Blümel, J; Eggers, Maren; Glebe, D; Rapp, I; von Rheinbaben, F; Sauerbrei, A; Steinmann, E; Steinmann, J; Willkommen, H; Wutzler, P; Rabenau, Holger F
In: Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz, Bd. 58, Nr. 4-5, S. 491–492, 2015, ISSN: 1436-9990.
@article{Schwebke.2015,
title = {Mitteilung der Deutschen Vereinigung zur Bek\"{a}mpfung der Viruskrankheiten e. V. (DVV) und des Robert Koch-Instituts (RKI) zur Ver\"{o}ffentlichung der aktualisierten Fassung der Leitlinie zur Pr\"{u}fung von chemischen Desinfektionsmitteln auf Wirksamkeit gegen Viren in der Humanmedizin (Suspensionstest) - Fassung vom 1. Dezember 2014},
author = {I Schwebke and J Bl\"{u}mel and Maren Eggers and D Glebe and I Rapp and F von Rheinbaben and A Sauerbrei and E Steinmann and J Steinmann and H Willkommen and P Wutzler and Holger F Rabenau},
doi = {10.1007/s00103-015-2130-9},
issn = {1436-9990},
year = {2015},
date = {2015-01-01},
journal = {Bundesgesundheitsblatt, Gesundheitsforschung, Gesundheitsschutz},
volume = {58},
number = {4-5},
pages = {491--492},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
