Fachinfo
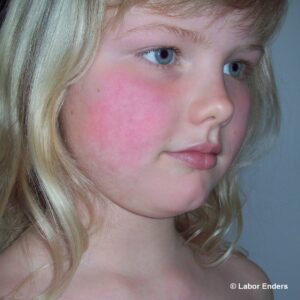
Das Parvovirus B19 verursacht die Ringelröteln, die wie Masern und Windpocken eine klassische „Kinderkrankheit“ sind. Meist verläuft diese Infektion bei Kindern und Erwachsenen harmlos. In der Schwangerschaft können jedoch schwere fetale Komplikationen auftreten.
Ansteckungsrisiko
Parvovirus B19-Infektionen treten gehäuft im Frühjahr bis zum Frühsommer auf. Häufigste Infektionsquelle für Schwangere sind infizierte Kinder im Kindergarten- und Grundschulalter. Die Ansteckung erfolgt durch Tröpfcheninfektion; das Ansteckungsrisiko ist bei Auftreten von Ringelröteln innerhalb der Familie am höchsten. Die Inkubationszeit liegt zwischen 13 und 18 Tagen. Infektiosität besteht ca eine Woche vor bis eine Woche nach Erkrankungsbeginn. In Deutschland haben 60–70 % der Erwachsenen Ringelröteln durchgemacht und so lebenslange Immunität erworben. Umgekehrt sind etwa 30–40 % der Frauen im gebärfähigen Alter nicht vor einer Parvovirus B19-Infektion geschützt.
Symptome
Charakteristisch ist das hauptsächlich bei Kindern auftretende Exanthem an den Wangen („Ohrfeigenkrankheit“) und ein girlandenförmiges Exanthem an Armen und Beinen. Bei Erwachsenen verläuft die Infektion in 30 % asymptomatisch, in 20% mit unspezifischen Symptomen und in 50 % mit Exanthem und/oder Gelenkbeschwerden.
Risiko in der Schwangerschaft
Eine Parvovirus B19 Infektion in der Schwangerschaft bleibt in der Mehrzahl der Fälle ohne Folgen für das werdende Kind. Eine fetale Infektion kann asymptomatisch verlaufen, aber auch zu Abort, Anämie und Hydrops fetalis führen. Letzterer ist die Folge einer Herzinsuffizienz bei schwerer anhaltender Anämie mit dem Risiko für einen intrauterinen Fruchttod. Das Hauptrisiko für einen Hydrops fetalis besteht bei einer mütterlicher Infektion in der 9.-20. SSW (ca. 7%). Die Abortrate („Excess-Risiko“) in der Frühschwangerschaft beträgt ca. 5 %.
Vorgehen bei akuter Infektion in der Schwangerschaft
- Es besteht keine Indikation zum Schwangerschaftsabbruch.
- Es sollte abhängig vom Gestationsalter eine engmaschige Überwachung durch Ultraschall- und Dopplerkontrollen (nicht-invasive Anämiediagnostik) erfolgen
- Bei unauffälligem Ultraschall/Doppler besteht keine Indikation für invasive Interventionen.
- Bei auffälligem Ultraschall/Doppler sollte die Indikation für eine invasive Pränataldiagnostik und fetale Therapie (intrauterine Transfusion) geprüft werden, da nach Beobachtungsstudien intrauterine Transfusionen die fetale Mortalität senken können.
Prävention
Es stehen weder ein Parvovirus B19-Impfstoff, noch spezifische Immunglobuline zur Verfügung. Die Expositionsprophylaxe ist schwierig, da das Ansteckungsrisiko vor Beginn des Exanthems am höchsten ist. Zudem treten bei infizierten Personen häufig keine Symptome auf.
Diagnostik
Wann sollte eine Parvovirus B19-Diagnostik in der Schwangerschaft erfolgen?
Nach Kontakt oder bei verdächtigen Symptomen sollten die IgG- und IgM-Antikörper (ggf. mit einem Zusatztest, wie z.B. IgG-Avidität, zur Eingrenzung des Infektionszeitpunktes) bestimmt werden. In der frühen Inkubationsphase (ca. 1 Woche nach Kontakt) kann mittels PCR Parvovirus B19-DNA in hoher Konzentration nachgewiesen werden.
- Wurde keine PCR in der frühen Inkubationphase durchgeführt und ist der IgG-, IgM-Befund negativ, sollte eine weitere Kontrolle in 2 Wochen zum Nachweis/Ausschluss einer Serokonversion erfolgen.
- Bei akuter Infektion (IgG und IgM positiv, PCR positiv, niedrige IgG-Avidität) werden engmaschige Ultraschall/Doppler-Kontrolle empfohlen. Das weitere Vorgehen ergibt sich aus diesen Befunden.
- Bei positivem IgG-, und negativem IgM-Befund ist Immunität anzunehmen. Liegt jedoch ein Hydrops vor, so muss beachtet werden, dass die IgM-Antikörper 6 bis 8 Wochen nach Infektion (in Abhängigkeit vom eingesetzten Test) auch unter die Nachweisgrenze fallen können. Es sollte in diesen Fällen eine PCR und Zusatzteste durchgeführt werden.
Wann sollte der Parvovirus B19-Immunstatus bestimmt werden?
Schwangere mit familiärem Kontakt zu Kindern unter sechs Jahren sollten ihren Immunstatus kennen oder möglichst früh in der Schwangerschaft überprüfen lassen. Diese Untersuchung ist nicht Gegenstand der Mutterschaftsvorsorge und somit IGeL. Bei beruflichem Kontakt zu Kleinkindern trägt der Arbeitgeber ggf. die Kosten für die Immunstatusbestimmung.
Für seronegative Schwangere besteht in den meisten Bundesländern ein Beschäftigungsverbot in vorschulischen Einrichtungen bis zur vollendeten 20. Schwangerschaftswoche.
Untersuchungsmaterial: Vollblut 2-3 ml, bei invasiver Pränataldiagnostik (Indikation s.o.): Fruchtwasser: 5-10 ml, Fetalblut: 0,5-1ml
Zum Herunterladen:
–> zur Übersicht: Prä- und perinatale Infektionen
Auswahl unserer Publikationen/Beiträge zur Parvovirus B19-Infektion
Enders, M; Kagan, KO
Infektionen in der Schwangerschaft und bei Geburt Buchkapitel
In: von Kaisenberg, C; Klaritsch, P; Hösli-Krais, I (Hrsg.): Die Geburtshilfe, S. 399-446, Springer, Berlin, Heidelberg, 6. Auflage, 2024, ISBN: 978-3-662-63506-3.
@inbook{Enders.2023,
title = {Infektionen in der Schwangerschaft und bei Geburt},
author = {M Enders and KO Kagan},
editor = {C von Kaisenberg and P Klaritsch and I H\"{o}sli-Krais},
doi = {10.1007/978-3-662-63506-3_64},
isbn = {978-3-662-63506-3},
year = {2024},
date = {2024-07-06},
urldate = {2024-07-06},
booktitle = {Die Geburtshilfe},
pages = {399-446},
publisher = {Springer, Berlin, Heidelberg},
edition = {6. Auflage},
series = {Springer Reference Medizin},
keywords = {},
pubstate = {published},
tppubtype = {inbook}
}
Beck, R; Exler, S; Enders, M
Parvovirus B19-Infektion und Schwangerschaft Artikel
In: Epid Bull, Nr. 24, S. 3–7, 2024.
@article{Beck.2024,
title = {Parvovirus B19-Infektion und Schwangerschaft},
author = {R Beck and S Exler and M Enders},
doi = {10.25646/12157},
year = {2024},
date = {2024-06-13},
urldate = {2024-06-13},
journal = {Epid Bull},
number = {24},
pages = {3\textendash7},
abstract = {Parvovirus B19, Schwangerschaft, Ausbruch, Epidemie, Ringelr\"{o}teln},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Eggers, M; Hübner, N; Blümel, J; Exner, M; Gebel, J; Helber-Soszynski, U; Ilschner, C; Rabenau, HF; Schwebke, I; Enders, M
In: Hygiene & Medizin, Bd. 49, Ausg. 6, 2024.
@article{Eggers.inpress,
title = {Gemeinsame Mitteilung von VAH und der Kommission Virusdesinfektion der DVV und GfV: Hygiene- und Desinfektionsma\ssnahmen bei Infektionen mit Parvovirus B19: Stand 15.05.2024},
author = {M Eggers and N H\"{u}bner and J Bl\"{u}mel and M Exner and J Gebel and U Helber-Soszynski and C Ilschner and HF Rabenau and I Schwebke and M Enders},
url = {https://www.vah-online.de
https://g-f-v.org/komissionen/},
year = {2024},
date = {2024-05-15},
urldate = {2024-05-15},
journal = {Hygiene \& Medizin},
volume = {49},
issue = {6},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Witteborn, K; Schoner, K; Enders, M; Gembruch, U; Arabin, B
In: Case Reports in perinatal medicine, Bd. 3, Nr. 1, S. 65–70, 2013.
@article{Witteborn.2013,
title = {Human parvovirus B19 infection causing discrepant prenatal findings and outcome in monochorionic diamniotic twins},
author = {K Witteborn and K Schoner and M Enders and U Gembruch and B Arabin},
doi = {10.1515/crpm-2013-0040},
year = {2013},
date = {2013-01-01},
urldate = {2013-01-01},
journal = {Case Reports in perinatal medicine},
volume = {3},
number = {1},
pages = {65--70},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Weiffenbach, J; Bald, R; Gloning, K P; Minderer, S; Gärtner, B C; Weidner, A; Hanke, M; Enders, M
In: The Journal of infectious diseases, Bd. 205, Nr. 5, S. 782–788, 2012.
@article{Weiffenbach.2012,
title = {Serological and virological analysis of maternal and fetal blood samples in prenatal human parvovirus B19 infection},
author = {J Weiffenbach and R Bald and K P Gloning and S Minderer and B C G\"{a}rtner and A Weidner and M Hanke and M Enders},
doi = {10.1093/infdis/jir855},
year = {2012},
date = {2012-01-01},
urldate = {2012-01-01},
journal = {The Journal of infectious diseases},
volume = {205},
number = {5},
pages = {782--788},
abstract = {BACKGROUND: Intrauterine parvovirus B19 (B19V) infection can be asymptomatic or may cause severe fetal complications. Information on serological and virological findings of infection in the fetus is scarce. METHODS: We determined B19V-DNA and anti-B19V antibodies in maternal and fetal blood samples obtained from 41 pregnancies that were complicated by prenatal B19V infection. Most fetuses presented with moderate to severe anemia or hydrops. RESULTS: At the time of fetal blood sampling, all mothers were B19V-DNA positive and B19V-IgG positive. B19V-IgM was detected in 95% of maternal blood samples. B19V-DNA, B19V-IgM, and B19V-IgG were detected in 100%, 28%, and 24% of fetal blood samples, respectively. The probability of a positive B19V-IgG or B19V-IgM finding in fetal blood increased with gestational age. B19V-IgG levels in maternal blood did not correlate with the likelihood of a positive B19V-IgG test in the fetus. The presence of B19V-IgG in fetal blood was accompanied by lower B19V-DNA levels and less severe clinical findings. CONCLUSIONS: The lack of B19V-IgG in fetuses with B19V-derived anemia or hydrops is most likely due to a limited materno-fetal transfer of IgG and a poor fetal antibody response. Fetal B19V infection is poorly controlled in the absence of specific antibodies},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Enders, M; Klingel, K; Weidner, A; Baisch, C; Kandolf, R; Schalasta, G; Enders, G
In: Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, Bd. 49, Nr. 3, S. 163–168, 2010.
@article{Enders.2010d,
title = {Risk of fetal hydrops and non-hydropic late intrauterine fetal death after gestational parvovirus B19 infection},
author = {M Enders and K Klingel and A Weidner and C Baisch and R Kandolf and G Schalasta and G Enders},
doi = {10.1016/j.jcv.2010.07.014},
year = {2010},
date = {2010-01-01},
urldate = {2010-01-01},
journal = {Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology},
volume = {49},
number = {3},
pages = {163--168},
abstract = {BACKGROUND: Risk assessment of parvovirus B19 (B19)-associated fetal complications following gestational B19 infection remains controversial. OBJECTIVES: To determine the risk of fetal hydrops or non-hydropic late intrauterine fetal death following acute maternal B19 infection at defined gestational weeks. STUDY DESIGN: Observational cohort study of pregnant women with serologic evidence of acute B19 infection. If available, fetal or neonatal tissue samples from cases complicated by fetal loss or hydrops were investigated for the presence of B19 DNA by polymerase chain reaction (PCR) and/or in situ hybridization (ISH). RESULTS: Of 236 women with known pregnancy outcome, 228 had a live birth and 8 a fetal loss. The observed rate of fetal hydrops for all pregnant women was 4.2% (10/236) (95% confidence interval [CI], 2.1-7.7) and 10.6% (10/94) (95% CI, 5.2-18.7) for those infected between 9 and 20 weeks gestation. Tissue samples from 8 hydrops cases were investigated by PCR or ISH and all were B19 DNA positive. Fetal death occurring during or after gestational week 22 was only observed in one case which was associated with B19-derived fetal hydrops. CONCLUSIONS: Our findings demonstrate that although adverse fetal outcome is a rare complication of gestational B19 infection, a relevant risk of fetal hydrops exists particularly for women infected between 9 and 20 weeks' gestation. Cases of B19-derived non-hydropic late intrauterine fetal death were not observed in the present study},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Schiesser, M; Sergi, C; Enders, M; Maul, H; Schnitzler, P
Discordant outcomes in a case of parvovirus b19 transmission into both dichorionic twins Artikel
In: Twin research and human genetics, Bd. 12, Nr. 2, S. 175–179, 2009.
@article{Schiesser.2009,
title = {Discordant outcomes in a case of parvovirus b19 transmission into both dichorionic twins},
author = {M Schiesser and C Sergi and M Enders and H Maul and P Schnitzler},
doi = {10.1375/twin.12.2.175},
year = {2009},
date = {2009-01-01},
urldate = {2009-01-01},
journal = {Twin research and human genetics},
volume = {12},
number = {2},
pages = {175--179},
abstract = {Maternal infection with parvovirus B19 during pregnancy can cause aplastic anemia in the fetus and may lead to nonimmune fetal hydrops and fetal demise. Twin pregnancies complicated by infection due to parvovirus B19 are very rare clinical events. We present a dichorionic, diamniotic, dizygotic twin pregnancy after in vitro fertilization with parvovirus B19 infection and viral transmission to both twins, but different outcomes. At 19 weeks gestation, hydrops fetalis was diagnosed for male twin A, female twin B did not show any abnormalities. At 22 weeks gestation an acute parvovirus B19 infection was detected and twin A was diagnosed with intrauterine fetal death (IUFD) by ultrasound at 23 weeks gestation. Viral DNA was detected in maternal blood as well as in placenta and liver tissue of this twin. Twin B was born at 35 weeks gestation asymptomatically and no signs of hydrops or other congenital anomalies but viral DNA was detected by PCR in serum. At the age of 2 years, both IgG titres against B19 and parvovirus DNA amplification copies were still positive in plasma of the surviving twin, but no clinical signs were detectable. It is remarkable that both twins were infected with parvovirus B19 early in pregnancy but showed a discordant clinical outcome. Our case report describes the rare occurrence of an intrauterine fetal death (IUFD) of one twin and the asymptomatic infection of the other in a twin pregnancy},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Enders, M; Weidner, A; Rosenthal, T; Baisch, C; Hedman, L; Söderlund-Venermo, M; Hedman, K
Improved diagnosis of gestational parvovirus B19 infection at the time of nonimmune fetal hydrops Artikel
In: The Journal of infectious diseases, Bd. 197, Nr. 1, S. 58–62, 2008.
@article{Enders.2008,
title = {Improved diagnosis of gestational parvovirus B19 infection at the time of nonimmune fetal hydrops},
author = {M Enders and A Weidner and T Rosenthal and C Baisch and L Hedman and M S\"{o}derlund-Venermo and K Hedman},
doi = {10.1086/524302},
year = {2008},
date = {2008-01-01},
urldate = {2008-01-01},
journal = {The Journal of infectious diseases},
volume = {197},
number = {1},
pages = {58--62},
abstract = {BACKGROUND: In the diagnosis of parvovirus B19 infection, the detection of virus-specific IgG in the absence of virus-specific IgM is considered to indicate past immunity. METHODS: We determined the diagnostic value of a high-quality B19 IgM EIA, compared with that of a VP1 IgG avidity EIA, a VP2 IgG epitope-type specificity (ETS) EIA, and real-time polymerase chain reaction (PCR) in the diagnosis of maternal B19 infection during nonimmune fetal hydrops. RESULTS: Serum samples from 101 pregnant women with confirmed B19-induced fetal hydrops were collected at the time of invasive prenatal diagnosis. The samples were investigated for B19 IgM, VP1 IgG avidity, and VP2 IgG ETS. With the B19 IgM EIA, 78 women (77.2 %) showed positive results, 15 (14.9%) showed negative results, and 8 (7.9 %) showed equivocal results. According to the combined B19 IgG avidity and IgG ETS EIA results, only 5 (5%) of 101 women were classified as having past immunity. Available serum samples (n = 24) that had nondiagnostic results in the antibody assays were further investigated by PCR. All were B19 DNA positive (mean load, 2.5 x 10(4) genome equivalents/mL; range, 2.5 x 10(3) - 7.8 x 10(6)). CONCLUSIONS: At the time of B19-induced hydrops, detection of B19 DNA in maternal blood had the best diagnostic sensitivity for identifying maternal B19 infection. However, given the long persistence of B19 DNAemia, supplementary measurement of VP1 IgG avidity and VP2 IgG ETS improves the precision of diagnosis and management of pregnant women affected by the B19 virus
~
BACKGROUND
In the diagnosis of parvovirus B19 infection, the detection of virus-specific IgG in the absence of virus-specific IgM is considered to indicate past immunity.
METHODS
We determined the diagnostic value of a high-quality B19 IgM EIA, compared with that of a VP1 IgG avidity EIA, a VP2 IgG epitope-type specificity (ETS) EIA, and real-time polymerase chain reaction (PCR) in the diagnosis of maternal B19 infection during nonimmune fetal hydrops.
RESULTS
Serum samples from 101 pregnant women with confirmed B19-induced fetal hydrops were collected at the time of invasive prenatal diagnosis. The samples were investigated for B19 IgM, VP1 IgG avidity, and VP2 IgG ETS. With the B19 IgM EIA, 78 women (77.2 %) showed positive results, 15 (14.9%) showed negative results, and 8 (7.9 %) showed equivocal results. According to the combined B19 IgG avidity and IgG ETS EIA results, only 5 (5%) of 101 women were classified as having past immunity. Available serum samples (n = 24) that had nondiagnostic results in the antibody assays were further investigated by PCR. All were B19 DNA positive (mean load, 2.5 x 10(4) genome equivalents/mL; range, 2.5 x 10(3) - 7.8 x 10(6)).
CONCLUSIONS
At the time of B19-induced hydrops, detection of B19 DNA in maternal blood had the best diagnostic sensitivity for identifying maternal B19 infection. However, given the long persistence of B19 DNAemia, supplementary measurement of VP1 IgG avidity and VP2 IgG ETS improves the precision of diagnosis and management of pregnant women affected by the B19 virus.
//
BACKGROUND
In the diagnosis of parvovirus B19 infection, the detection of virus-specific IgG in the absence of virus-specific IgM is considered to indicate past immunity.
METHODS
We determined the diagnostic value of a high-quality B19 IgM EIA, compared with that of a VP1 IgG avidity EIA, a VP2 IgG epitope-type specificity (ETS) EIA, and real-time polymerase chain reaction (PCR) in the diagnosis of maternal B19 infection during nonimmune fetal hydrops.
RESULTS
Serum samples from 101 pregnant women with confirmed B19-induced fetal hydrops were collected at the time of invasive prenatal diagnosis. The samples were investigated for B19 IgM, VP1 IgG avidity, and VP2 IgG ETS. With the B19 IgM EIA, 78 women (77.2 %) showed positive results, 15 (14.9%) showed negative results, and 8 (7.9 %) showed equivocal results. According to the combined B19 IgG avidity and IgG ETS EIA results, only 5 (5%) of 101 women were classified as having past immunity. Available serum samples (n = 24) that had nondiagnostic results in the antibody assays were further investigated by PCR. All were B19 DNA positive (mean load, 2.5 x 10(4) genome equivalents/mL; range, 2.5 x 10(3) - 7.8 x 10(6)).
CONCLUSIONS
At the time of B19-induced hydrops, detection of B19 DNA in maternal blood had the best diagnostic sensitivity for identifying maternal B19 infection. However, given the long persistence of B19 DNAemia, supplementary measurement of VP1 IgG avidity and VP2 IgG ETS improves the precision of diagnosis and management of pregnant women affected by the B19 virus.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
~
BACKGROUND
In the diagnosis of parvovirus B19 infection, the detection of virus-specific IgG in the absence of virus-specific IgM is considered to indicate past immunity.
METHODS
We determined the diagnostic value of a high-quality B19 IgM EIA, compared with that of a VP1 IgG avidity EIA, a VP2 IgG epitope-type specificity (ETS) EIA, and real-time polymerase chain reaction (PCR) in the diagnosis of maternal B19 infection during nonimmune fetal hydrops.
RESULTS
Serum samples from 101 pregnant women with confirmed B19-induced fetal hydrops were collected at the time of invasive prenatal diagnosis. The samples were investigated for B19 IgM, VP1 IgG avidity, and VP2 IgG ETS. With the B19 IgM EIA, 78 women (77.2 %) showed positive results, 15 (14.9%) showed negative results, and 8 (7.9 %) showed equivocal results. According to the combined B19 IgG avidity and IgG ETS EIA results, only 5 (5%) of 101 women were classified as having past immunity. Available serum samples (n = 24) that had nondiagnostic results in the antibody assays were further investigated by PCR. All were B19 DNA positive (mean load, 2.5 x 10(4) genome equivalents/mL; range, 2.5 x 10(3) - 7.8 x 10(6)).
CONCLUSIONS
At the time of B19-induced hydrops, detection of B19 DNA in maternal blood had the best diagnostic sensitivity for identifying maternal B19 infection. However, given the long persistence of B19 DNAemia, supplementary measurement of VP1 IgG avidity and VP2 IgG ETS improves the precision of diagnosis and management of pregnant women affected by the B19 virus.
//
BACKGROUND
In the diagnosis of parvovirus B19 infection, the detection of virus-specific IgG in the absence of virus-specific IgM is considered to indicate past immunity.
METHODS
We determined the diagnostic value of a high-quality B19 IgM EIA, compared with that of a VP1 IgG avidity EIA, a VP2 IgG epitope-type specificity (ETS) EIA, and real-time polymerase chain reaction (PCR) in the diagnosis of maternal B19 infection during nonimmune fetal hydrops.
RESULTS
Serum samples from 101 pregnant women with confirmed B19-induced fetal hydrops were collected at the time of invasive prenatal diagnosis. The samples were investigated for B19 IgM, VP1 IgG avidity, and VP2 IgG ETS. With the B19 IgM EIA, 78 women (77.2 %) showed positive results, 15 (14.9%) showed negative results, and 8 (7.9 %) showed equivocal results. According to the combined B19 IgG avidity and IgG ETS EIA results, only 5 (5%) of 101 women were classified as having past immunity. Available serum samples (n = 24) that had nondiagnostic results in the antibody assays were further investigated by PCR. All were B19 DNA positive (mean load, 2.5 x 10(4) genome equivalents/mL; range, 2.5 x 10(3) - 7.8 x 10(6)).
CONCLUSIONS
At the time of B19-induced hydrops, detection of B19 DNA in maternal blood had the best diagnostic sensitivity for identifying maternal B19 infection. However, given the long persistence of B19 DNAemia, supplementary measurement of VP1 IgG avidity and VP2 IgG ETS improves the precision of diagnosis and management of pregnant women affected by the B19 virus.
Enders, M; Weidner, A; Enders, G
In: Epidemiology and infection, Bd. 135, Nr. 4, S. 563–569, 2007.
@article{Enders.2007e,
title = {Current epidemiological aspects of human parvovirus B19 infection during pregnancy and childhood in the western part of Germany},
author = {M Enders and A Weidner and G Enders},
doi = {10.1017/S095026880600731X},
year = {2007},
date = {2007-01-01},
urldate = {2007-01-01},
journal = {Epidemiology and infection},
volume = {135},
number = {4},
pages = {563--569},
abstract = {This investigation was undertaken to provide detailed information on the epidemiology of human parvovirus B19 (B19) infection during pregnancy and childhood in the western part of Germany. Between 1997 and 2004, 40,517 sera from pregnant women aged 17-45 years and 6060 sera from children and young adults were tested for B19 IgG and IgM in our laboratory. In pregnant women, both the history of a 'specific' (OR 7.7, 95% CI 5.2-11.4) and a 'non-specific' rash (OR 3.3, 95% CI 1.5-7.1) was predictive for B19 IgM positivity. The B19 IgG prevalence was 69.2% (4097/5924) in a subgroup of asymptomatic pregnant women screened for B19 antibodies. In children, the age-specific IgG-positivity rate increased from 12.2% (66/541) at 2 years of age to 71.9% (396/551) in those older than 10 years. In conclusion, the prevalence of B19 IgG in pregnant women from the western part of Germany is higher then previously reported. Contact with children aged 3-10 years is a major risk factor for exposure to B19. Pregnant women with the history of a 'non-specific' rash should also be evaluated for acute B19 infection},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Enders, M; Schalasta, G; Baisch, C; Weidner, A; Pukkila, L; Kaikkonen, L; Lankinen, H; Hedman, L; Söderlund-Venermo, M; Hedman, K
In: Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology, Bd. 35, Nr. 4, S. 400–406, 2006.
@article{Enders.2006,
title = {Human parvovirus B19 infection during pregnancy--value of modern molecular and serological diagnostics},
author = {M Enders and G Schalasta and C Baisch and A Weidner and L Pukkila and L Kaikkonen and H Lankinen and L Hedman and M S\"{o}derlund-Venermo and K Hedman},
doi = {10.1016/j.jcv.2005.11.002},
year = {2006},
date = {2006-01-01},
urldate = {2006-01-01},
journal = {Journal of clinical virology : the official publication of the Pan American Society for Clinical Virology},
volume = {35},
number = {4},
pages = {400--406},
abstract = {BACKGROUND
Over 95% of fetal complications (fetal hydrops and death) occur within 12 weeks following acute parvovirus B19 (B19) infection in pregnancy. Therefore, weekly fetal ultrasound monitoring is generally recommended for this time period. However, in the majority of women, typical symptoms of acute infection (rash or arthropathy) are absent, and during epidemics, B19 infection may be diagnosed incidentally by antibody screening of women at risk.
OBJECTIVE
To assess the diagnostic value of currently available molecular and serological methods for reliable diagnosis of primary B19 infection in pregnancy.
STUDY DESIGN
Large panels of well-characterized acute-phase or convalescent sera were used to investigate the ability of a VP2 IgM EIA, a Light-Cycler-based B19-DNA PCR, a VP1-IgG avidity EIA and two VP2-IgG epitope-type specificity [ETS] EIAs to pinpoint the time of primary B19 infection in pregnancy.
RESULTS
The duration of low-level IgM positivity varied greatly (range 4-26 weeks). Samples collected within the first 2 weeks of infection showed high-level viremia (mean 1.75 x 10(8) geq/ml). During follow-up, low-level DNAemia (mean 9.7 x 10(4)geq/ml) persisted for at least 18 weeks in 91% (20/22) of patients. Considering the first 12 weeks after onset of disease the window of greatest risk for fetal complications, the textquotedblacutetextquotedbl phase was extended to cover this full period. In this case, performing the avidity and ETS-EIA sequentially, the positive predictive value was 100% in patients showing concordant avidity and ETS-EIA results.
CONCLUSIONS
In the presence of low IgM titres and/or low-level DNAemia the use of supplementary serological assays such as VP1-IgG avidity EIA and VP2-ETS-EIA is advisable for restriction or avoidance of unnecessary fetal ultrasound examinations or invasive diagnostics; and in general for strengthening the reliability of B19 serodiagnosis of pregnant women.
~
BACKGROUND
Over 95% of fetal complications (fetal hydrops and death) occur within 12 weeks following acute parvovirus B19 (B19) infection in pregnancy. Therefore, weekly fetal ultrasound monitoring is generally recommended for this time period. However, in the majority of women, typical symptoms of acute infection (rash or arthropathy) are absent, and during epidemics, B19 infection may be diagnosed incidentally by antibody screening of women at risk.
OBJECTIVE
To assess the diagnostic value of currently available molecular and serological methods for reliable diagnosis of primary B19 infection in pregnancy.
STUDY DESIGN
Large panels of well-characterized acute-phase or convalescent sera were used to investigate the ability of a VP2 IgM EIA, a Light-Cycler-based B19-DNA PCR, a VP1-IgG avidity EIA and two VP2-IgG epitope-type specificity [ETS] EIAs to pinpoint the time of primary B19 infection in pregnancy.
RESULTS
The duration of low-level IgM positivity varied greatly (range 4-26 weeks). Samples collected within the first 2 weeks of infection showed high-level viremia (mean 1.75 x 10(8) geq/ml). During follow-up, low-level DNAemia (mean 9.7 x 10(4)geq/ml) persisted for at least 18 weeks in 91% (20/22) of patients. Considering the first 12 weeks after onset of disease the window of greatest risk for fetal complications, the textquotedblacutetextquotedbl phase was extended to cover this full period. In this case, performing the avidity and ETS-EIA sequentially, the positive predictive value was 100% in patients showing concordant avidity and ETS-EIA results.
CONCLUSIONS
In the presence of low IgM titres and/or low-level DNAemia the use of supplementary serological assays such as VP1-IgG avidity EIA and VP2-ETS-EIA is advisable for restriction or avoidance of unnecessary fetal ultrasound examinations or invasive diagnostics; and in general for strengthening the reliability of B19 serodiagnosis of pregnant women.
//
BACKGROUND
Over 95% of fetal complications (fetal hydrops and death) occur within 12 weeks following acute parvovirus B19 (B19) infection in pregnancy. Therefore, weekly fetal ultrasound monitoring is generally recommended for this time period. However, in the majority of women, typical symptoms of acute infection (rash or arthropathy) are absent, and during epidemics, B19 infection may be diagnosed incidentally by antibody screening of women at risk.
OBJECTIVE
To assess the diagnostic value of currently available molecular and serological methods for reliable diagnosis of primary B19 infection in pregnancy.
STUDY DESIGN
Large panels of well-characterized acute-phase or convalescent sera were used to investigate the ability of a VP2 IgM EIA, a Light-Cycler-based B19-DNA PCR, a VP1-IgG avidity EIA and two VP2-IgG epitope-type specificity [ETS] EIAs to pinpoint the time of primary B19 infection in pregnancy.
RESULTS
The duration of low-level IgM positivity varied greatly (range 4-26 weeks). Samples collected within the first 2 weeks of infection showed high-level viremia (mean 1.75 x 10(8) geq/ml). During follow-up, low-level DNAemia (mean 9.7 x 10(4)geq/ml) persisted for at least 18 weeks in 91% (20/22) of patients. Considering the first 12 weeks after onset of disease the window of greatest risk for fetal complications, the textquotedblacutetextquotedbl phase was extended to cover this full period. In this case, performing the avidity and ETS-EIA sequentially, the positive predictive value was 100% in patients showing concordant avidity and ETS-EIA results.
CONCLUSIONS
In the presence of low IgM titres and/or low-level DNAemia the use of supplementary serological assays such as VP1-IgG avidity EIA and VP2-ETS-EIA is advisable for restriction or avoidance of unnecessary fetal ultrasound examinations or invasive diagnostics; and in general for strengthening the reliability of B19 serodiagnosis of pregnant women.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Over 95% of fetal complications (fetal hydrops and death) occur within 12 weeks following acute parvovirus B19 (B19) infection in pregnancy. Therefore, weekly fetal ultrasound monitoring is generally recommended for this time period. However, in the majority of women, typical symptoms of acute infection (rash or arthropathy) are absent, and during epidemics, B19 infection may be diagnosed incidentally by antibody screening of women at risk.
OBJECTIVE
To assess the diagnostic value of currently available molecular and serological methods for reliable diagnosis of primary B19 infection in pregnancy.
STUDY DESIGN
Large panels of well-characterized acute-phase or convalescent sera were used to investigate the ability of a VP2 IgM EIA, a Light-Cycler-based B19-DNA PCR, a VP1-IgG avidity EIA and two VP2-IgG epitope-type specificity [ETS] EIAs to pinpoint the time of primary B19 infection in pregnancy.
RESULTS
The duration of low-level IgM positivity varied greatly (range 4-26 weeks). Samples collected within the first 2 weeks of infection showed high-level viremia (mean 1.75 x 10(8) geq/ml). During follow-up, low-level DNAemia (mean 9.7 x 10(4)geq/ml) persisted for at least 18 weeks in 91% (20/22) of patients. Considering the first 12 weeks after onset of disease the window of greatest risk for fetal complications, the textquotedblacutetextquotedbl phase was extended to cover this full period. In this case, performing the avidity and ETS-EIA sequentially, the positive predictive value was 100% in patients showing concordant avidity and ETS-EIA results.
CONCLUSIONS
In the presence of low IgM titres and/or low-level DNAemia the use of supplementary serological assays such as VP1-IgG avidity EIA and VP2-ETS-EIA is advisable for restriction or avoidance of unnecessary fetal ultrasound examinations or invasive diagnostics; and in general for strengthening the reliability of B19 serodiagnosis of pregnant women.
~
BACKGROUND
Over 95% of fetal complications (fetal hydrops and death) occur within 12 weeks following acute parvovirus B19 (B19) infection in pregnancy. Therefore, weekly fetal ultrasound monitoring is generally recommended for this time period. However, in the majority of women, typical symptoms of acute infection (rash or arthropathy) are absent, and during epidemics, B19 infection may be diagnosed incidentally by antibody screening of women at risk.
OBJECTIVE
To assess the diagnostic value of currently available molecular and serological methods for reliable diagnosis of primary B19 infection in pregnancy.
STUDY DESIGN
Large panels of well-characterized acute-phase or convalescent sera were used to investigate the ability of a VP2 IgM EIA, a Light-Cycler-based B19-DNA PCR, a VP1-IgG avidity EIA and two VP2-IgG epitope-type specificity [ETS] EIAs to pinpoint the time of primary B19 infection in pregnancy.
RESULTS
The duration of low-level IgM positivity varied greatly (range 4-26 weeks). Samples collected within the first 2 weeks of infection showed high-level viremia (mean 1.75 x 10(8) geq/ml). During follow-up, low-level DNAemia (mean 9.7 x 10(4)geq/ml) persisted for at least 18 weeks in 91% (20/22) of patients. Considering the first 12 weeks after onset of disease the window of greatest risk for fetal complications, the textquotedblacutetextquotedbl phase was extended to cover this full period. In this case, performing the avidity and ETS-EIA sequentially, the positive predictive value was 100% in patients showing concordant avidity and ETS-EIA results.
CONCLUSIONS
In the presence of low IgM titres and/or low-level DNAemia the use of supplementary serological assays such as VP1-IgG avidity EIA and VP2-ETS-EIA is advisable for restriction or avoidance of unnecessary fetal ultrasound examinations or invasive diagnostics; and in general for strengthening the reliability of B19 serodiagnosis of pregnant women.
//
BACKGROUND
Over 95% of fetal complications (fetal hydrops and death) occur within 12 weeks following acute parvovirus B19 (B19) infection in pregnancy. Therefore, weekly fetal ultrasound monitoring is generally recommended for this time period. However, in the majority of women, typical symptoms of acute infection (rash or arthropathy) are absent, and during epidemics, B19 infection may be diagnosed incidentally by antibody screening of women at risk.
OBJECTIVE
To assess the diagnostic value of currently available molecular and serological methods for reliable diagnosis of primary B19 infection in pregnancy.
STUDY DESIGN
Large panels of well-characterized acute-phase or convalescent sera were used to investigate the ability of a VP2 IgM EIA, a Light-Cycler-based B19-DNA PCR, a VP1-IgG avidity EIA and two VP2-IgG epitope-type specificity [ETS] EIAs to pinpoint the time of primary B19 infection in pregnancy.
RESULTS
The duration of low-level IgM positivity varied greatly (range 4-26 weeks). Samples collected within the first 2 weeks of infection showed high-level viremia (mean 1.75 x 10(8) geq/ml). During follow-up, low-level DNAemia (mean 9.7 x 10(4)geq/ml) persisted for at least 18 weeks in 91% (20/22) of patients. Considering the first 12 weeks after onset of disease the window of greatest risk for fetal complications, the textquotedblacutetextquotedbl phase was extended to cover this full period. In this case, performing the avidity and ETS-EIA sequentially, the positive predictive value was 100% in patients showing concordant avidity and ETS-EIA results.
CONCLUSIONS
In the presence of low IgM titres and/or low-level DNAemia the use of supplementary serological assays such as VP1-IgG avidity EIA and VP2-ETS-EIA is advisable for restriction or avoidance of unnecessary fetal ultrasound examinations or invasive diagnostics; and in general for strengthening the reliability of B19 serodiagnosis of pregnant women.
Schorling, S; Schalasta, G; Enders, G; Zauke, M
In: The Journal of molecular diagnostics : JMD, Bd. 6, Nr. 1, S. 37–41, 2004.
@article{Schorling.2004,
title = {Quantification of parvovirus B19 DNA using COBAS AmpliPrep automated sample preparation and LightCycler real-time PCR},
author = {S Schorling and G Schalasta and G Enders and M Zauke},
doi = {10.1016/S1525-1578(10)60489-8},
year = {2004},
date = {2004-01-01},
urldate = {2004-01-01},
journal = {The Journal of molecular diagnostics : JMD},
volume = {6},
number = {1},
pages = {37--41},
abstract = {The COBAS AmpliPrep instrument (Roche Diagnostics GmbH, D-68305 Mannheim, Germany) automates the entire sample preparation process of nucleic acid isolation from serum or plasma for polymerase chain reaction analysis. We report the analytical performance of the LightCycler Parvovirus B19 Quantification Kit (Roche Diagnostics) using nucleic acids isolated with the COBAS AmpliPrep instrument. Nucleic acids were extracted using the Total Nucleic Acid Isolation Kit (Roche Diagnostics) and amplified with the LightCycler Parvovirus B19 Quantification Kit. The kit combination processes 72 samples per 8-hour shift. The lower detection limit is 234 IU/ml at a 95% hit-rate, linear range approximately 10(4)-10(10) IU/ml, and overall precision 16 to 40%. Relative sensitivity and specificity in routine samples from pregnant women are 100% and 93%, respectively. Identification of a persistent parvovirus B19-infected individual by the polymerase chain reaction among 51 anti-parvovirus B19 IgM-negative samples underlines the importance of additional nucleic acid testing in pregnancy and its superiority to serology in identifying the risk of parvovirus B19 transmission via blood or blood products. Combination of the Total Nucleic Acid Isolation Kit on the COBAS AmpliPrep instrument with the LightCycler Parvovirus B19 Quantification Kit provides a reliable and time-saving tool for sensitive and accurate detection of parvovirus B19 DNA},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Enders, M; Weidner, A; Zoellner, I; Searle, K; Enders, G
In: Prenatal diagnosis, Bd. 24, Nr. 7, S. 513–518, 2004.
@article{Enders.2004,
title = {Fetal morbidity and mortality after acute human parvovirus B19 infection in pregnancy: prospective evaluation of 1018 cases},
author = {M Enders and A Weidner and I Zoellner and K Searle and G Enders},
doi = {10.1002/pd.940},
year = {2004},
date = {2004-01-01},
urldate = {2004-01-01},
journal = {Prenatal diagnosis},
volume = {24},
number = {7},
pages = {513--518},
abstract = {OBJECTIVE: To determine more precisely the incidence of fetal complications following maternal parvovirus B19 infection at various gestational ages. METHODS: An observational prospective study of 1018 pregnant women whose acute B19 infection was serologically confirmed in our laboratory. RESULTS: The observed rate of fetal death throughout pregnancy was 6.3% (64/1018) (95% confidence interval [CI]: 4.9, 8.0). The fetal death rate for those infected within the first 20 weeks of gestation (WG) was 64/579 (11.0%). Fetal death was only observed when maternal B19 infection occurred before the completed 20 WG. The observed stillbirth proportion was 0.6% (6/960). Three of six stillbirth cases presented with fetal hydrops. The overall risk of hydrops fetalis was 3.9% (40/1018) (95% CI: 2.8, 5.3). Three of 17 cases with non-severe hydrops and 13 of 23 cases with severe hydrops received intrauterine transfusion(s). The proportion of fetuses with severe hydrops that survived following fetal transfusions was 11/13 (84.6%). All of the non-transfused fetuses with severe hydrops died. CONCLUSION: Our data demonstrate a relevant B19-associated risk of fetal death, which is largely confined to maternal B19 infection in the first 20 WG. Timely intrauterine transfusion of fetuses with severe hydrops fetalis reduces the risk of fetal death. Parvovirus B19-associated stillbirth without hydropic presentation is not a common finding},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
